Pavilion Publishing and Media Ltd
Blue Sky Offices Shoreham, 25 Cecil Pashley Way, Shoreham-by-Sea, West Sussex, BN43 5FF, UNITED KINGDOM
Every year, there are roughly 50 million cases of sepsis worldwide, with one in five (11 million) dying from the disease annually.1
Sepsis is more common than heart disease, stroke and cancer, and is a leading cause of death in hospitals, yet it is significantly underfunded compared to these other disease areas.
Each year, the US spends $2,277m on cancer research, $1,236m on heart disease and $317m on stroke care, but just $91m on sepsis (Figure 1).
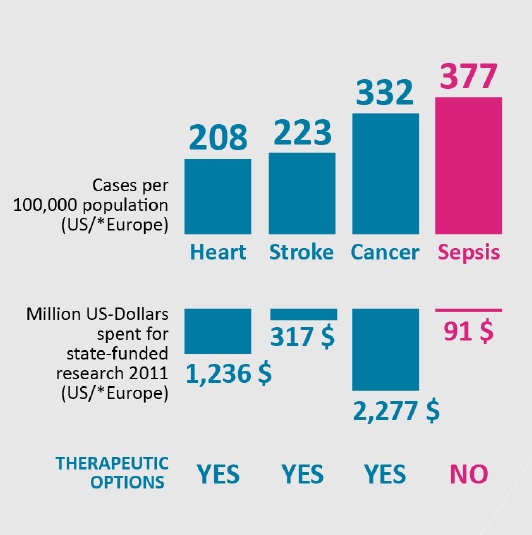
A lack of funding in sepsis research has meant there is currently no therapeutic, individualised treatment for sepsis patients. Sepsis bundles are available to guide clinicians, but frontline staff find this guidance difficult to translate into practice, and are often left to exercise their own clinical judgement when assessing and managing sepsis.2
However, new research has established that sepsis can be caused by the excessive production of neutrophil extracellular traps (NETs), and this is paving the way for new diagnostic tools and treatment options.
Diagnosing sepsis
The Center for Disease Control and Prevention (CDC) defines sepsis as: “The body’s extreme response to an infection.” Bacterial infections cause most cases of sepsis, but it can also be caused by viral infections (such as Covid-19) and fungal infections.3
Certain groups are at higher risk of sepsis including adults aged 65 and over, children under one, people with a weakened immune system, those with chronic medical conditions and hospitalised people.3
Sepsis can be divided into three stages: sepsis, severe sepsis and septic shock. If it is not treated quickly, it can lead to tissue damage, organ malfunction, organ failure and death.
However, sepsis is challenging to recognise in its early stages, as many of its symptoms are also common in other conditions. These include fever, chills, rapid breathing and heart rate, breathleness, confusion, and disorientation.4
Clinicians can conduct a complete blood count (a common blood test) to identify the markers of sepsis, such as a high or low white blood cell count, high levels of lactic acids and the presence of C-reactive protein.5
A blood culture test may also be conducted to identify what type of bacteria or fungi caused infection in the blood, as well as urine, saliva and tissue samples and imaging tests (such as X-rays, CT scans and MRI scans) to find the source of infection.
However, none of these tests can diagnose sepsis, and the results must be combined with information about the illness and a physical examination to help clinicians determine whether their patient is septic.5
Treating sepsis
Generally, UK healthcare workers now follow the ‘Sepsis Six’ care bundle, which instructs clinicians to carry out six tasks (including oxygen, cultures, antibiotics, fluids, lactate measurement and urine output monitoring) within a timeframe of one hour.6
Guidelines have altered significantly over the last two decades, and it is now widely believed that if a doctor suspects sepsis, treatment should begin right away, without waiting for test results.
In 2004, there were two sepsis bundles: a resuscitation bundle and a management bundle, each to be completed within six and 24 hours respectively. In 2012, the Surviving Sepsis Campaign (SSC) revised these bundles and replaced them with 3- and 6-Hour Bundles to ensure treatment was administered more quickly.7
Now, both these bundles have been combined into a 1-Hour Bundle, and the goal is to begin both resuscitation and management immediately, as longer delays may lead to higher mortality.7
The 1-Hour Bundle instructs clinicians to:
- Measure lactate level
- Obtain blood cultures
- Administer broad-spectrum antibiotics
- Begin rapid administration of 30ml/kg crystalloid for hypotension or lactate
- Apply vasopressors if hypotensive during or after fluid resuscitation to maintain a mean arterial pressure > 65mm Hg.
However, this latest revision has come under criticism for various reasons, including that large volumes of fluid may be harmful to administer, measuring serum lactate concentrations is not proven to improve patient outcomes, and that a 1-hour target may over-diagnose patients with sepsis.8
Furthermore, these bundles are very generalised tools that do not offer patients any personalised treatment options, and now, researchers are working to create ways to treat sepsis patients in a more individualised manner.
Why are there still no therapeutic options for sepsis?
Sepsis can leave patients with lifechanging side effects, with half of survivors left with sequelae. The long-term side effects of sepsis may prevent people from returning to work, and they may become reliant on carers or family members to support them in daily activities.9
Yet sepsis is still significantly underfunded, and this means there are still no specific sepsis treatments, according to Professor Dijilali Annane, Professor of Medicine, University Paris Saclay-UVSQ.
He explains: “We have treatments for cancer, and there are many treatments for cardiovascular disease and stroke, but there aren’t any treatments for sepsis, and this is an immediate consequence of an under-recognised problem that has not received enough investment.”
Prof Annane says that while there is a profusion of high-quality papers on sepsis, including clinical trials, clinical research, microbiology and immunology, there is no collaboration across the fields, and this is significantly holding up progress.
“This means we are treating septic patients in a generic manner, without individualisation of each patient’s care. We are relying on a central lab with a slow turnaround on test results, which is slowing down clinicians’ ability to make the right treatment decision,” he said.
By the next decade, Prof Annane hopes hospitals will have integrated a number of new tools to transform routine care. This includes ‘digital twins’, which will help to anticipate the patient’s disease trajectory and whether they will respond to certain treatments.
However, more work needs to be done to address some of the key challenges, such as assessing severity, creating a more personalised approach to treatment and creating an anti-NETs treatment, he says.
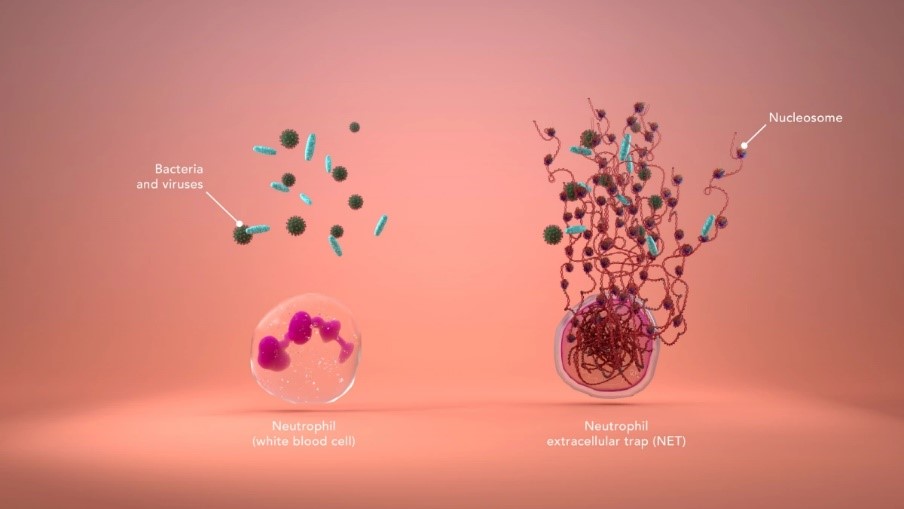
What are NETs?
NETs are sticky webs made of long strips of nucleosomes (Figure 2). The webs are produced by neutrophils – the most abundant type of white blood cell – when the body detects bacteria or viruses.
NETs are designed to stop the infection from spreading around the body, and are therefore an important part of the body’s immune response. However, “the presence of too many of them in the blood can tip the immune system’s delicate balance from reaction to overreaction,” explains Dr Jake Micallef, Chief Scientific Officer, Volition.
Excessive production of NETs can lead to tissue damage, the formation of microthrombi, and in severe cases, sepsis, shock and death. Elevated levels of NETs are also associated with poor patient outcomes in Covid-19 and cancer, says Dr Micallef.
Research performed early on in the Covid pandemic found that levels of H3.1 nucleosomes were significantly raised in patients with severe Covid. This was an indicator that NETs may have a role in Covid outcomes, and it has now been shown that in severe Covid-19 cases, excessive production of NETs in the lungs can lead to severe lung impairment or death.10
NETs were only discovered in 2004, and since their discovery, researchers have been closely analysing the role of NETs in various disease areas. Now, they are recognised as a major driver in diseases which cause millions of deaths every year.
Researchers are now working to create new ways of detecting the early stages of NETosis and treatments which could cleanse NETs from the blood, something which could have far-reaching consequences in clinical practice, according to Dr Andrew Aswani, Consultant in Intensive Care Medicine and Anaesthesia at Guy’s and St Thomas’ NHS Foundation Trust.
“NETs are such a critical part of the inflammation pathway, they are relevant in any inflammatory disease, whether that’s thrombosis, Covid-19, sepsis, cancer, and transplants. The unique and powerful potential of measuring them – and potentially reducing them – could really empower human health,” he said.
A new diagnostic tool
Leading experts in epigenetics have now developed a new technology that can detect NETs in minute quantities. The Nu.Q test uses a simple, routine blood test, allowing clinicians to catch diseases like sepsis and cancer early, and improve patients’ chances of successful treatment.11
The test also allows clinicians to predict disease severity, monitor disease progression and response to treatment, enabling physicians to rapidly triage patients depending on their results.
The test has recently been approved for use in Europe in both ELISA (Enzyme-Linked Immunosorbent Assay) and automated ChLIA (ChemiLuminescence ImmunoAssay) formats, and Dr Micallef says Volition is now undertaking large-scale studies across multiple sites in the US to determine clinical utility in sepsis and support an application to the FDA’s Breakthrough Device programme.15
Could a NETs test pave the way for a new sepsis treatment?
A new treatment designed to capture and remove circulating NETs from patient blood, without compromising the defensive function of neutrophils, is also being developed.
The NucleoCapture device has completed a First-in-Man clinical study designed to assess the safety and efficacy of therapeutic apheresis in patients with sepsis and septic shock, which showed that a single pass of NET and cfDNA-contaminated blood through the device results in over 95% removal of NETs.12
The technology has now been granted designation as a Breakthrough Device by The Center for Devices and Radiological Health (CDRH) of the FDA, and additional clinical trials are planned for the US and Europe on sepsis and transplantation, with hopes of achieving subsequent CE Marking in the near future.
This article is based on a talk given by Dr Jake Micallef, Professor Dijilali Annane and Dr Andrew Retter given at a Media Education Session at the International Symposium on Intensive Care and Emergency Medicine (ISICEM) in Brussels.
- Dr Jake Micallef is Chief Scientific Officer, Volition
- Professor Dijilali Annane is Professor of Medicine, University Paris Saclay-UVSQ
- Dr Andrews Retter is Specialist in Intensive Care, ECMO and Thrombosis and Guy’s and St Thomas’
References
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet (London, England). 2020;395(10219):200-11.
- NHS England. Sepsis guidance implementation advice for adults. 2017. Available at: https://www.england.nhs.uk/wp-content/uploads/2017/09/sepsis-guidance-implementation-advice-for-adults.pdf
- Centers for Disease Control and Prevention (CDC). What is sepsis? 2022. Available at: https://www.cdc.gov/sepsis/what-is-sepsis.html
- NHS Inform. Sepsis. 2023. Available at: https://www.nhsinform.scot/illnesses-and-conditions/blood-and-lymph/sepsis
- Sepsis Alliance. Testing for sepsis. 2022. Available at: https://www.sepsis.org/sepsis-basics/testing-for-sepsis/
- Frankling C, Patel J, Sharif B, Melody T, Yeung J, Gao F, et al. A Snapshot of Compliance with the Sepsis Six Care Bundle in Two Acute Hospitals in the West Midlands, UK. Indian J Crit Care Med 2019;23(7):310–315.
- Barochia AV, Cui X, Eichacker PQ. The Surviving Sepsis Campaign’s Revised Sepsis Bundles. Curr Infect Dis Rep. 2013 Oct;15(5):385-93. doi: 10.1007/s11908-013-0351-3. PMID: 23990342; PMCID: PMC3864669.
- Sepsis Program Optimization. Evolution and Current Status of Sepsis Bundles. 2020. Available at: https://sepsisprogramoptimization.com/spo-bundles
- Sepsis Alliance. Post-sepsis syndrome (PSS). 2021. Available at: https://www.sepsis.org/sepsis-basics/post-sepsis-syndrome/
- Cavalier E, Guiot J, Lechner K, Dutsch A, Eccleston M, Herzog M, Bygott T, Schomburg A, Kelly T, Holdenrieder S. Circulating Nucleosomes as Potential Markers to Monitor COVID-19 Disease Progression. Front Mol Biosci. 2021 Mar 18;8:600881. doi: 10.3389/fmolb.2021.600881. PMID: 33816549; PMCID: PMC8012533.
- Nu.Q NETs. Monitoring the immune system to save lives. Available at: https://volition.com/nu-q-nets
- Santersus NucleoCapture Technology Designated as a Breakthrough Device for the Treatment of Sepsis by the US FDA. Santersus. 2022. Available at: https://santersus.com/santersus-nucleocapture-technology-designated-as-a-breakthrough-device-for-the-treatment-of-sepsis-by-the-us-fda/


